The World Health Organization (WHO) has taken a historic step by releasing its first-ever global guidelines for the use of a specific class of weight-loss medications. For the first time, the WHO has provided official recommendations on using Glucagon-Like Peptide-1 (GLP-1) receptor agonists to manage obesity, which it classifies as a chronic and relapsing disease.
A Global Health Crisis Demands Action
In a significant blog post published on 1 December, the global health body underscored the severe impact of obesity worldwide. The WHO pointed out that a staggering 3.7 million deaths in 2024 were linked to this condition. The organization issued a stark warning, stating that if decisive and widespread action is not taken, this devastating figure could potentially double by the year 2030.
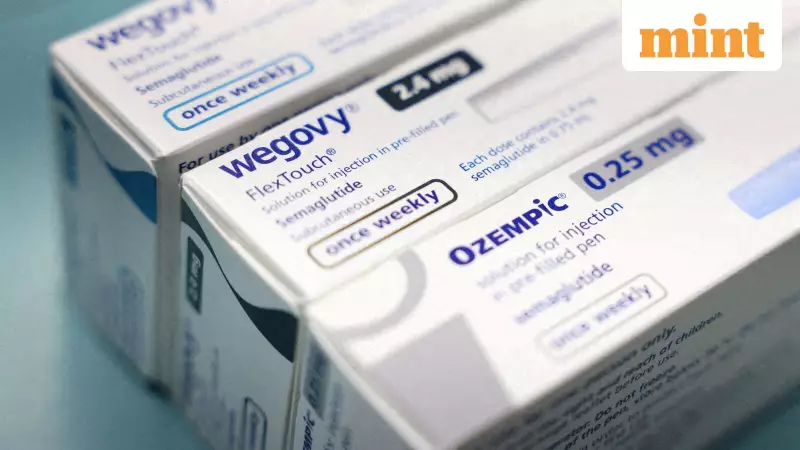
GLP-1 therapies, which include drugs like semaglutide and liraglutide, are not entirely new to the WHO's radar. They were previously included in the WHO's Essential Medicines List for managing type-2 diabetes, specifically for high-risk patient groups. The new guideline marks a pivotal expansion, formally recommending these drugs to address the "serious health challenge" of obesity itself.
However, the WHO strongly emphasises that these medications are not a standalone magic bullet. They are intended to be part of a comprehensive, holistic treatment plan. Dr. Tedros Adhanom Ghebreyesus, the WHO Director-General, clarified this approach. He stated, "While medication alone won’t solve this global health crisis, GLP-1 therapies can help millions overcome obesity and reduce its associated harms." The guidelines consistently recommend combining medication with healthy diet, increased physical activity, and ongoing consultation with healthcare professionals.
Key Recommendations and Cautions from WHO
The WHO's new guidance centres on two primary recommendations for the use of GLP-1 drugs in adults living with obesity.
First, the guidelines state that these medicines may be used by adults (excluding pregnant women) for the long-term management of obesity. This recommendation, however, comes with a major caveat: it is conditional. The WHO notes that data on the long-term side effects of these therapies remains limited. Other significant hurdles include the high cost of treatment, challenges in maintaining the regimen over a lifetime, and the current lack of preparedness in many health systems to deliver such care equitably.
Second, the WHO advises that adults prescribed GLP-1 treatments should also be offered intensive behavioural interventions. These interventions focus on supporting healthy eating habits and regular physical activity. This suggestion is based on what the WHO terms "low-certainty evidence," which indicates that combining drugs with lifestyle changes might improve overall treatment outcomes.
Australian Regulator Adds Crucial Mental Health Warning
As the global conversation around GLP-1 drugs intensifies, a significant safety alert has emerged from Australia. The Therapeutic Goods Administration (TGA), the country's drug regulator, has issued a new warning for patients using GLP-1 receptor agonists like Ozempic, Wegovy, Saxenda, Trulicity, and Mounjaro.
The TGA has advised doctors to closely monitor patients for the emergence or worsening of depression, suicidal thoughts, or any unusual changes in mood or behaviour. This warning was prompted by dozens of reports linking these drugs to suicidal behaviour and ideation. Importantly, the regulator clarified that a clear causal link has not yet been definitively established, but the potential risk warrants caution.
In a related update, the label for the drug Mounjaro (tirzepatide) now carries an additional warning. It alerts female patients that the medication may reduce the effectiveness of oral contraceptive pills, advising the use of alternative or backup contraceptive methods.
The simultaneous release of the WHO's treatment guidelines and new safety warnings highlights the complex, evolving landscape of obesity pharmacotherapy. It underscores a global push to address a deadly epidemic while rigorously managing the potential risks associated with powerful new treatments.