The Food and Drugs Administration (FDA) of Haryana has issued an urgent public safety alert concerning a specific batch of a children's cough syrup found to be adulterated with a toxic industrial chemical.
Contaminated Batch Identified and Recalled
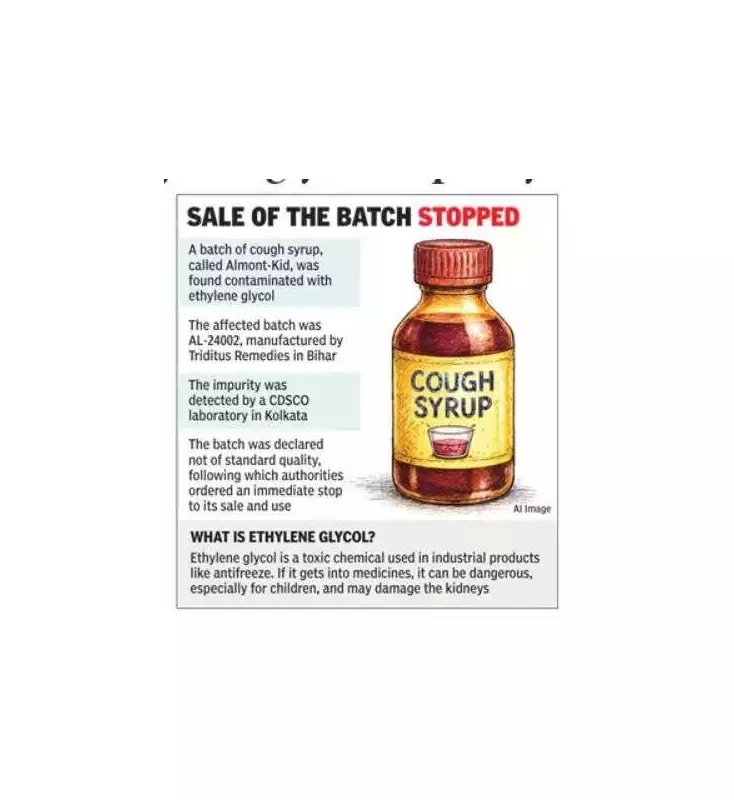
In an official order dated January 9, 2025, the state drugs controller declared the batch "not of standard quality." The product in question is Almont-Kid (Levocetirizine Dihydrochloride and Montelukast Sodium Syrup), bearing batch number AL-24002. It was manufactured by M/s Triditus Remedies at its facility in Phase-II Industrial Area, Hajipur, Bihar.
The syrup has a manufacturing date of January 2025 and an expiry date of December 2026. The FDA has directed an immediate halt to the sale and distribution of this batch across Haryana.
Source of Contamination and Health Risks
The alert was triggered after the Haryana FDA received an official report from the Central Drugs Standard Control Organisation (CDSCO), East Zone, Kolkata. Laboratory analysis revealed the presence of ethylene glycol (EG) impurity in the syrup at levels exceeding permissible safety limits.
Ethylene glycol is a toxic chemical commonly used in industrial products like antifreeze. It is not meant for human consumption and is strictly regulated in medicines. Health experts warn that exposure to high levels of EG, especially in children, can lead to severe health complications, including acute kidney damage and other systemic failures.
The regulatory order, however, does not mention any specific adverse incident reports linked to this batch in Haryana so far.
Immediate Actions and Public Advisory
The FDA's directive mandates immediate action from all stakeholders in the drug supply chain. The department has instructed:
- Retailers, wholesalers, and distributors to stop the sale and distribution of batch AL-24002.
- Hospitals and medical practitioners to neither prescribe nor use this batch.
- All pharmacies and hospitals to withdraw any remaining stock from their shelves and warehouses immediately.
Consumers and caregivers have been strongly advised to check the batch number on the label of any Almont-Kid syrup they possess. If the batch number matches AL-24002, they should avoid using it entirely. The public is also urged to cooperate with enforcement teams during inspections.
This incident highlights the ongoing vigilance by drug regulatory authorities to monitor product quality and protect public health from substandard and adulterated medicines.