Hims & Hers Abruptly Withdraws Copycat Wegovy Pill Amid Regulatory Pressure
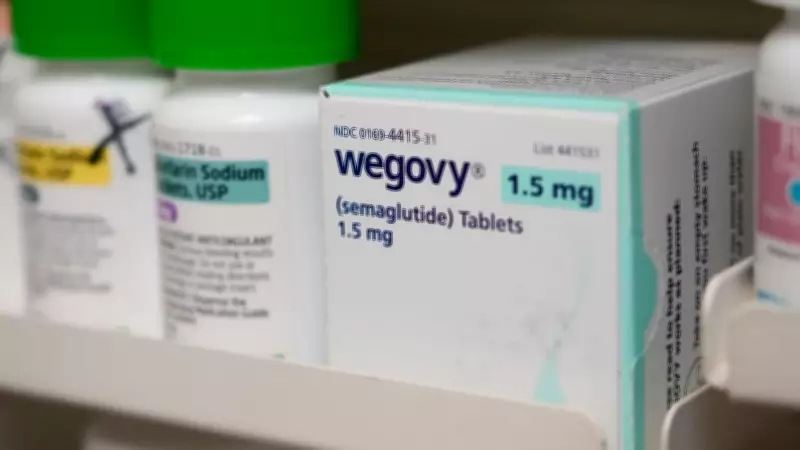
In a dramatic reversal, United States-based telehealth and pharmaceuticals company Hims & Hers Health Inc. has announced it will cease selling its copycat version of Novo Nordisk's blockbuster Wegovy weight-loss pill. This decision comes merely two days after the product's controversial launch, according to a detailed report from Bloomberg News.
FDA Crackdown and Stakeholder Pressure Force Quick Reversal
The move follows increased scrutiny from the US Food and Drug Administration, which has been actively cracking down on copycat weight-loss treatments in the pharmaceutical market. In a social media post on platform X (formerly Twitter) dated February 7, Hims stated that the decision resulted from constructive conversations with stakeholders. Notably, this post has since been removed from the company's social media page.
This development represents a significant victory for Novo Nordisk, which had previously accused Hims of selling an unapproved, inauthentic and untested product. In a defiant post on February 5, Hims had rejected these allegations, emphasizing its commitment to accessibility and a customer-first approach in healthcare delivery.
Timeline of a Controversial Product Launch
The controversy began on Thursday when Hims announced plans to sell a cheaper alternative to Novo Nordisk's Wegovy pill, which analysts had hailed as one of the most successful drug launches in recent years. Novo Nordisk immediately responded by declaring the move illegal and threatening legal action against the telehealth company.
The situation escalated rapidly when the FDA announced an investigation into the matter the following day. Adding to the regulatory pressure, the Department of Health and Human Services referred Hims to the Department of Justice for potential violations of federal law regarding pharmaceutical compounding and distribution practices.
Broader Industry Context and Historical Friction
This incident highlights the ongoing tension between major pharmaceutical companies and telehealth providers over compounded weight-loss medications. Novo Nordisk and Eli Lilly & Co., the makers of leading GLP-1 drugs, have repeatedly complained that the FDA has not done enough to prevent the proliferation of cheaper, compounded alternatives to their patented medications.
This marks the first instance where Hims has withdrawn a knockoff product following a formal complaint from a pharmaceutical company. The practice of telehealth companies selling compounded weight-loss drugs began during supply shortages several years ago and has persisted despite the resolution of those shortages.
Failed Partnership and Ongoing Disputes
The relationship between Hims and Novo Nordisk has been contentious for some time. Last year, the two companies attempted to collaborate on selling discounted weight-loss injections, but the partnership dissolved after just a few months, partly due to disagreements over compounding practices.
We had an agreement that the mass compounding would stop and unfortunately it didn't stop, stated Ludovic Helfgott, executive vice president of product and portfolio strategy at Novo Nordisk, referencing the failed collaboration.
Hims CEO Andrew Dudum had previously taken a defiant stance, declaring he wouldn't cave to pharmaceutical company demands regarding copycat weight-loss drugs. However, the combined pressure from regulatory authorities and industry stakeholders has now prompted this significant policy reversal.
The FDA has declined to comment on the specific case, while representatives for Novo Nordisk did not immediately respond to requests for comment regarding Hims' decision to withdraw the product from the market.