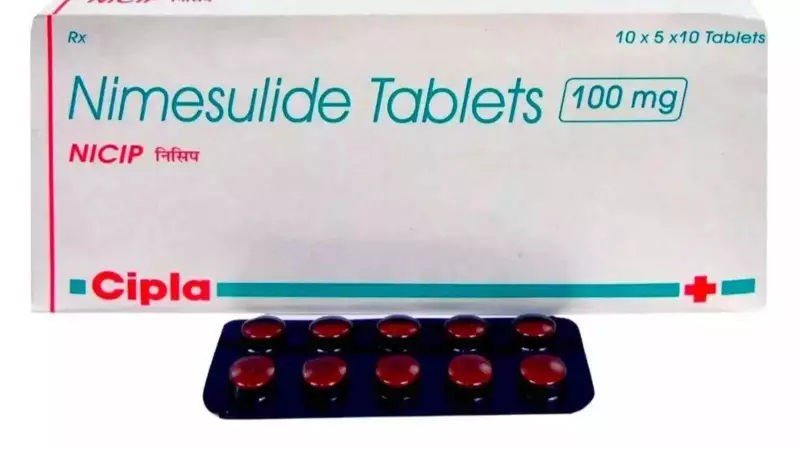
The Indian government has taken a decisive step to protect public health by imposing an immediate ban on all oral formulations of the painkiller nimesulide that exceed 100 milligrams in immediate-release form. This action targets the manufacture, sale, and distribution of these higher-dose tablets, citing significant safety concerns and the existence of safer medical alternatives.
Official Notification and Legal Basis
The prohibition was formally announced through a notification issued on December 29 and published in The Gazette of India. The Union Health Ministry stated it was convinced that the continued use of nimesulide in doses above 100mg posed a "likely risk to human beings." The ban, deemed necessary in the public interest, was enacted under Section 26A of the Drugs and Cosmetics Act, 1940, following consultations with the Drugs Technical Advisory Board (DTAB).
It is crucial to note that the restriction applies specifically to immediate-release oral tablets and capsules with a strength greater than 100mg. Lower-dose formulations and other forms of the drug, such as gels or suspensions, are not covered by this order. The directive takes effect immediately, compelling all manufacturers, distributors, and retailers to halt production and supply of the banned formulations.
A Drug Under Long-Standing Scrutiny
Nimesulide, a popular non-steroidal anti-inflammatory drug (NSAID) used to treat pain, inflammation, and fever, has been under the regulatory lens for over a decade. In 2011, the central government, acting on DTAB advice, prohibited six drugs and specifically banned nimesulide use in children below 12 years due to fears of liver toxicity.
Earlier this year, the Indian Council of Medical Research (ICMR) reinforced these safety worries. The premier medical body recommended restricting nimesulide use in patients below 18 and above 60 years and explicitly advised a ban on formulations exceeding 100mg. In the Indian market, the drug is widely sold under brand names like Nimulid, Nimtex, Nicip, and Nise, with the latter being one of the top-selling brands.
Medical Community Welcomes Safety-First Move
The government's decision has been welcomed by healthcare professionals who have long advocated for stricter controls on the drug. Dr. Rommel Tickoo, Director of Internal Medicine at Max Hospital, Saket, praised the move as prudent and timely.
"This is a prudent and timely decision taken by the government," Dr. Tickoo said. "The restriction on high-dose immediate-release nimesulide addresses a long-standing safety concern, especially when misused or taken without supervision. With safer NSAID alternatives readily available, limiting exposure to higher doses is clearly in the interest of patient safety."
This ban underscores a growing emphasis on pharmacovigilance and evidence-based regulation in India's pharmaceutical landscape. It directs both the medical community and the public towards therapeutic options with a better safety profile, aiming to reduce preventable drug-related adverse effects.